奥林匹亚:新靶向疗法对转移性乳腺癌伴基因突变患者优于标准化疗
乳腺癌易感基因(BRCA)是一种肿瘤抑制基因,发生突变后即失去肿瘤抑制作用,对侧乳腺癌和异时卵巢癌的风险增加。大约5%的未经筛选乳腺癌患者携带种系BRCA突变,种系是多细胞动物能繁殖后代的一类细胞总称,包括单倍体配子以及最终能分化成配子的原始生殖细胞,又称:性细胞系、生殖细胞系。该突变较多见于有明确乳腺癌家族史的患者、年轻患者、三阴性乳腺癌患者等人群。BRCA1突变患者多为三阴性乳腺癌,而BRCA2突变患者多为雌激素受体阳性乳腺癌。在体外,BRCA1或BRCA2突变细胞对多腺苷二磷酸核糖聚合酶(PARP)抑制剂敏感。奥拉帕利(奥拉帕尼)是一种口服的PARP抑制剂,已被临床证实对复发性卵巢癌伴种系BRCA突变的患者有效而获批。
2017年6月4日,美国麻省(马萨诸塞州)医学会《新英格兰医学杂志》在线发表纪念斯隆凯特林癌症中心、韩国首尔国立大学、波兰格但斯克医科大学、中国医学科学院北京协和医学院国家癌症中心(徐兵河)、吉林大学白求恩第一医院(李薇)、美国宾夕法尼亚大学、日本国立病院机构大阪医疗中心、法国古斯塔夫鲁西研究所、美国贝斯以色列女执事医疗中心、达纳法伯哈佛癌症中心、英国曼彻斯特大学、克里斯蒂医院、英国阿斯利康、美国阿斯利康、意大利威尼托肿瘤研究所、帕多瓦大学的奥林匹亚研究(OlympiAD)报告全文,发现奥拉帕利单药与医生选择的标准化疗方案相比,对HER2阴性转移性乳腺癌伴种系BRCA突变患者更安全有效。
OlympiAD (NCT02000622): Assessment of the Efficacy and Safety of Olaparib Monotherapy Versus Physicians Choice Chemotherapy in the Treatment of Metastatic Breast Cancer Patients With Germline BRCA1/2 Mutations.
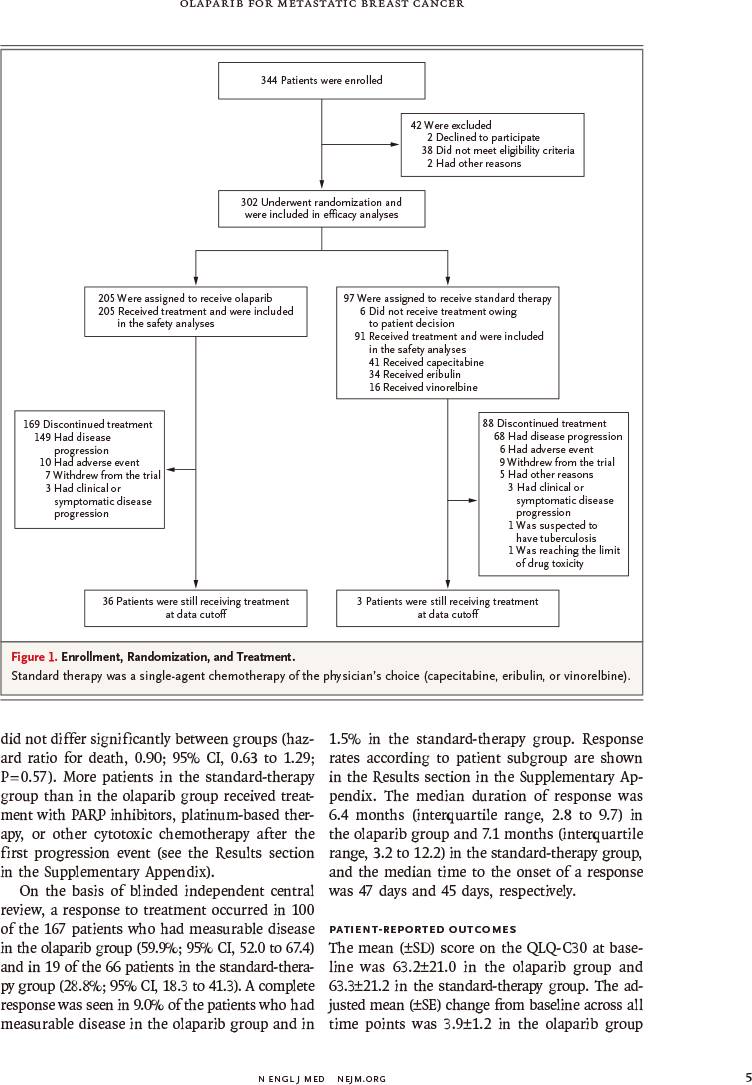
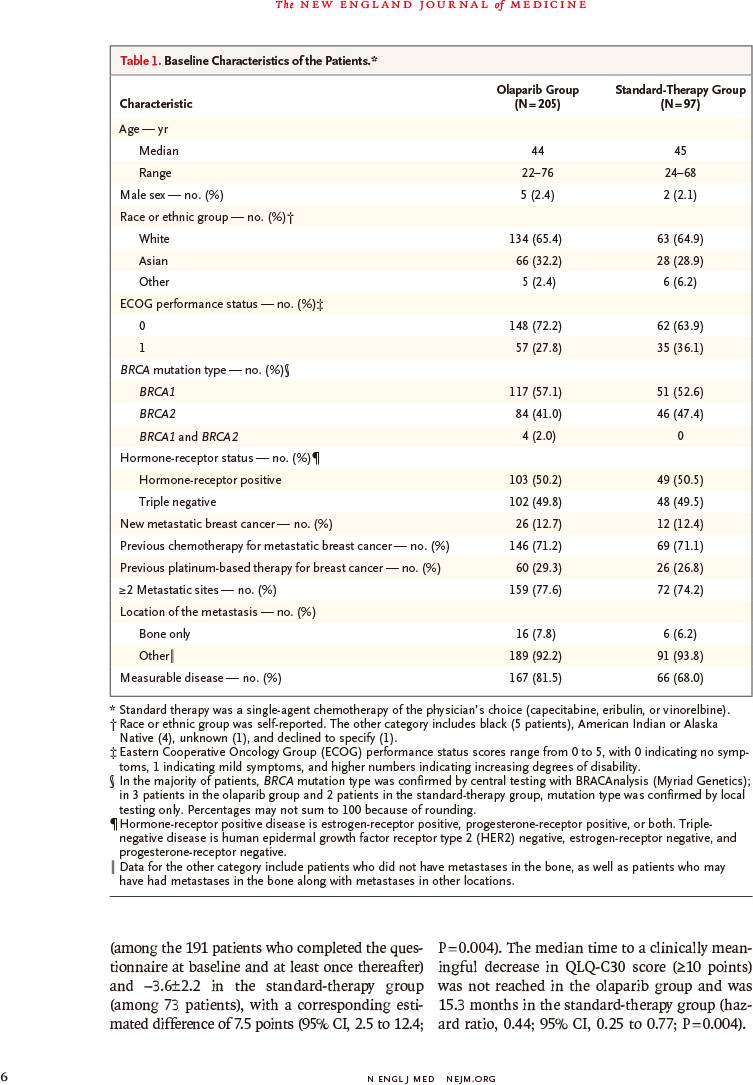
该随机非盲Ⅲ期研究于2014年4月7日~2015年11月27日入组302例既往接受过≤2种化疗方案的HER2阴性转移性乳腺癌伴种系BRCA突变患者,以2∶1的比例随机分配,接受奥拉帕利片剂(300mg,每天2次)或医生选择的单药化疗标准疗法(卡培他滨、艾日布林、长春瑞滨,每21天)。主要终点为无进展生存,通过盲法由独立中心进行评定,并根据意向治疗进行分析。
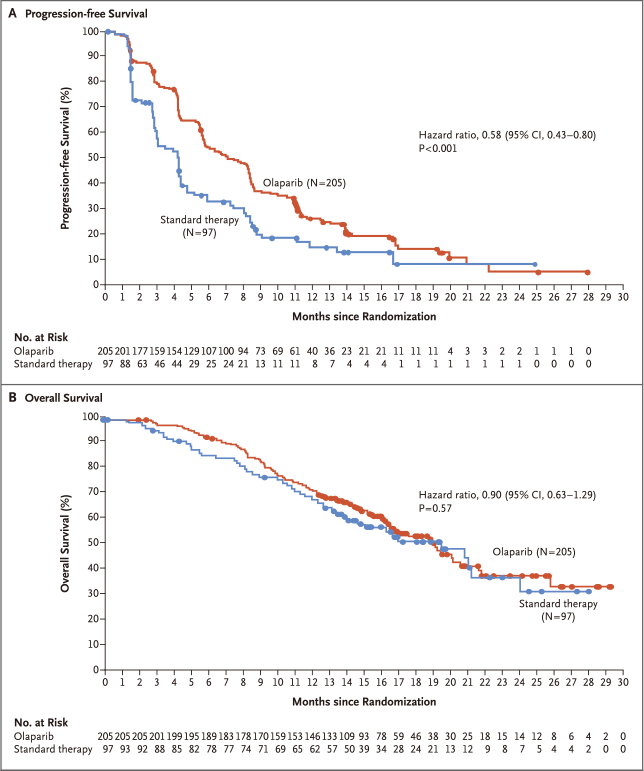
结果发现,奥拉帕利(205例)与标准疗法(97例)相比:
-
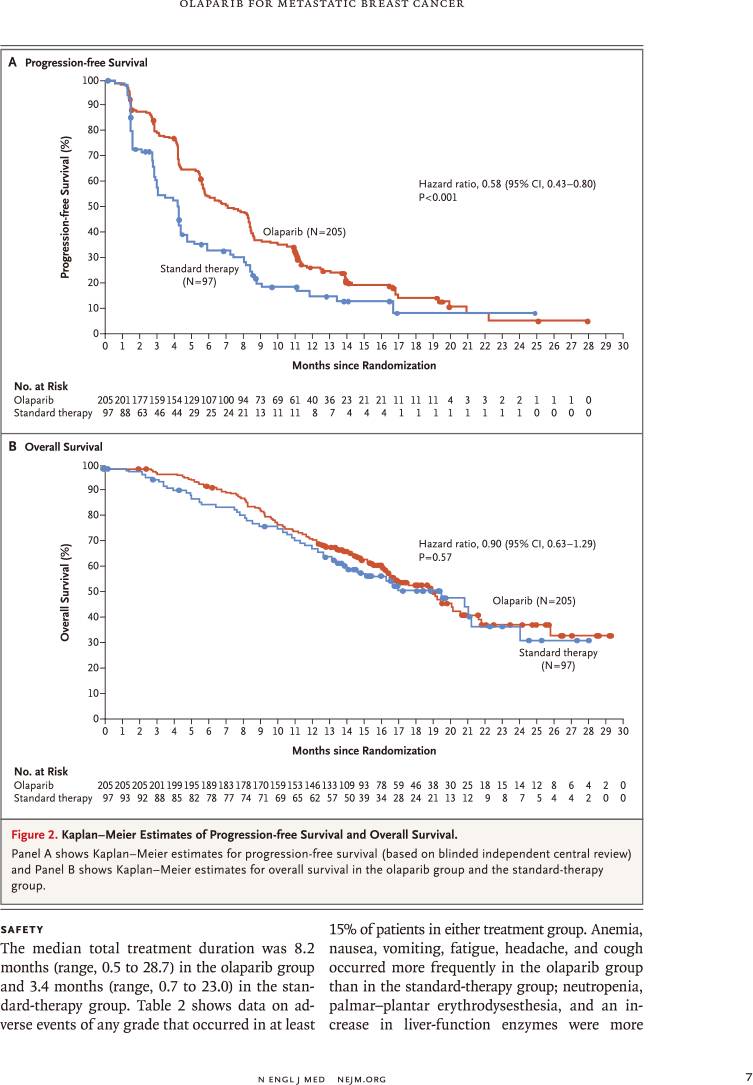
中位无进展生存显著延长2.8个月(7.0个月比4.2个月)
-
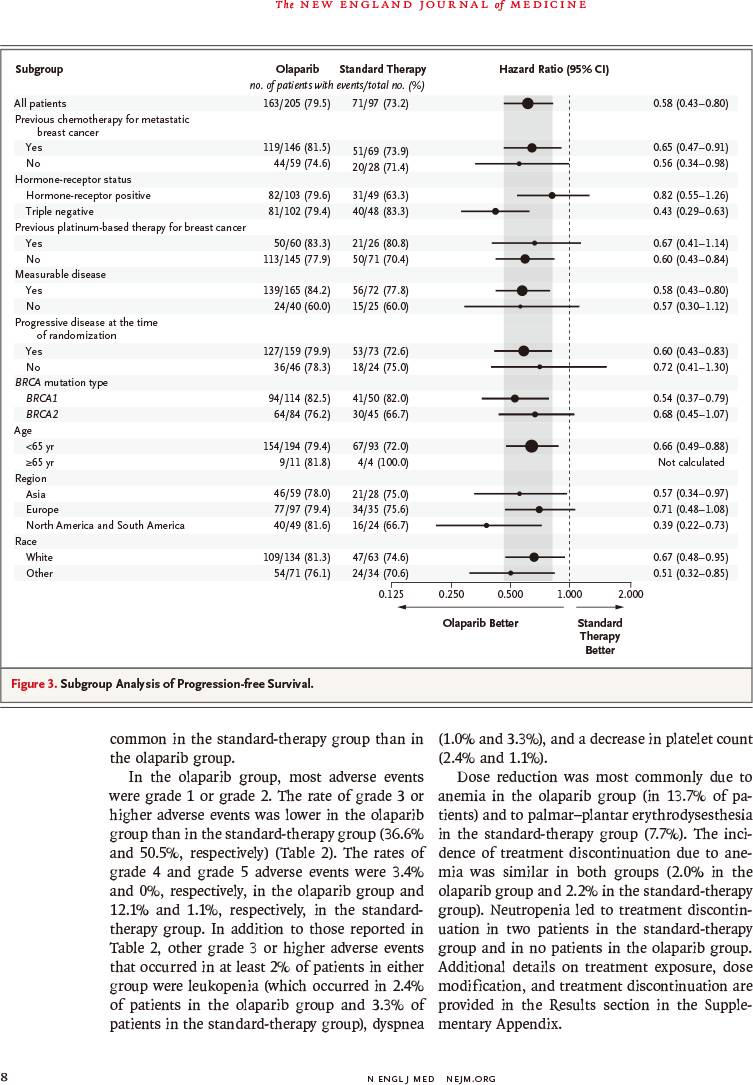
疾病进展或死亡风险降低42%(风险比:0.58,95%置信区间:0.43~0.80,P<0.001)
-
死亡风险降低10%(风险比:0.90,95%置信区间:0.63~1.29,P<0.57)
-
缓解率较高(59.9%比28.8%)
-
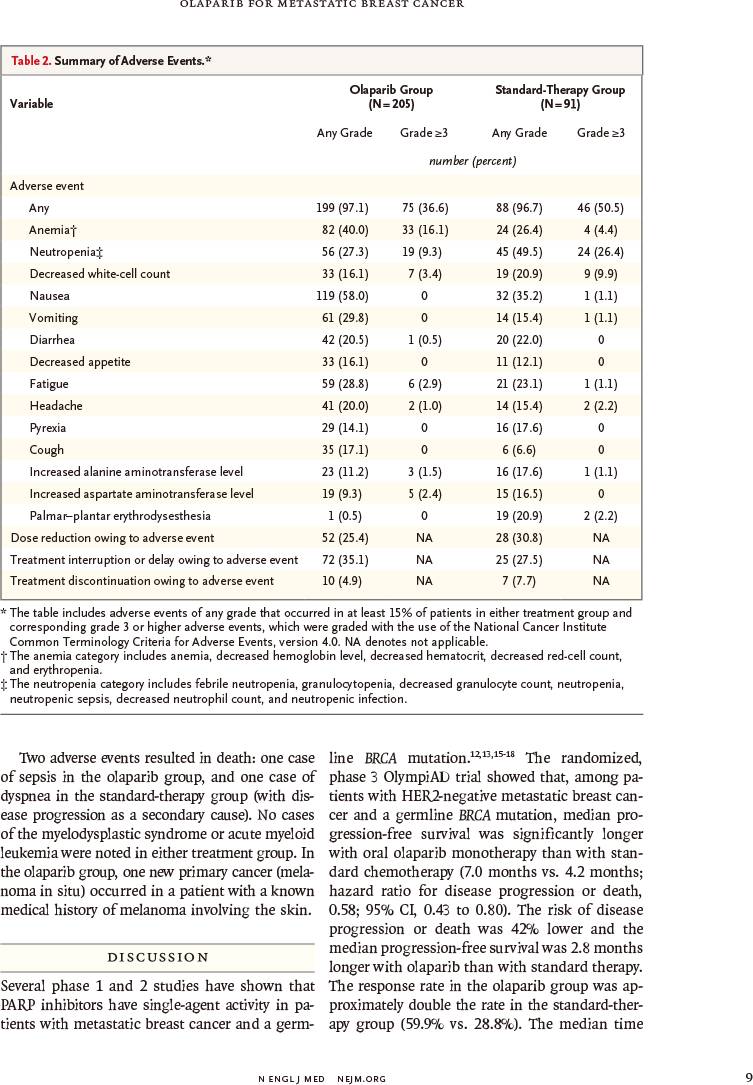
≥3级不良事件发生率较低(36.6%比50.5%)
-
毒性反应所致治疗中止率较低(4.9%比7.7%)
因此,对于HER2阴性转移性乳腺癌伴种系BRCA突变患者,奥拉帕利单药治疗与标准疗法相比,提供显著获益;中位无进展生存延长2.8个月,疾病进展或死亡风险降低42%。
N Engl J Med. 2017 Jun 4. [Epub ahead of print]
Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation.
Mark Robson, Seock-Ah Im, Elzbieta Senkus, Binghe Xu, Susan M. Domchek, Norikazu Masuda, Suzette Delaloge, Wei Li, Nadine Tung, Anne Armstrong, Wenting Wu, Carsten Goessl, Sarah Runswick, Pierfranco Conte.
Memorial Sloan Kettering Cancer Center, New York; Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, South Korea; Medical University of Gdańsk, Gdańsk, Poland; National Cancer Center-Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing; First Hospital of Jilin University, Changchun, in China; Basser Center, University of Pennsylvania, Philadelphia; National Hospital Organization, Osaka National Hospital, Osaka, Japan; Institut Gustave Roussy, Villejuif, France; Beth Israel Deaconess Medical Center, Dana-Farber Harvard Cancer Center, Boston; Christie Hospital NHS Foundation Trust, Faculty of Biology, Medicine and Health, University of Manchester, Manchester; AstraZeneca, Macclesfield, United Kingdom; AstraZeneca, Gaithersburg, MD; University of Padua, Istituto Oncologico Veneto Istituto di Ricovero e Cura a Carattere Scientifico, Padua, Italy.
BACKGROUND: Olaparib is an oral poly(adenosine diphosphate-ribose) polymerase inhibitor that has promising antitumor activity in patients with metastatic breast cancer and a germline BRCA mutation.
METHODS: We conducted a randomized, open-label, phase 3 trial in which olaparib monotherapy was compared with standard therapy in patients with a germline BRCA mutation and human epidermal growth factor receptor type 2 (HER2)-negative metastatic breast cancer who had received no more than two previous chemotherapy regimens for metastatic disease. Patients were randomly assigned, in a 2:1 ratio, to receive olaparib tablets (300 mg twice daily) or standard therapy with single-agent chemotherapy of the physician's choice (capecitabine, eribulin, or vinorelbine in 21-day cycles). The primary end point was progression-free survival, which was assessed by blinded independent central review and was analyzed on an intention-to-treat basis.
RESULTS: Of the 302 patients who underwent randomization, 205 were assigned to receive olaparib and 97 were assigned to receive standard therapy. Median progression-free survival was significantly longer in the olaparib group than in the standard-therapy group (7.0 months vs. 4.2 months; hazard ratio for disease progression or death, 0.58; 95% confidence interval, 0.43 to 0.80; P<0.001). The response rate was 59.9% in the olaparib group and 28.8% in the standard-therapy group. The rate of grade 3 or higher adverse events was 36.6% in the olaparib group and 50.5% in the standard-therapy group, and the rate of treatment discontinuation due to toxic effects was 4.9% and 7.7%, respectively.
CONCLUSIONS: Among patients with HER2-negative metastatic breast cancer and a germline BRCA mutation, olaparib monotherapy provided a significant benefit over standard therapy; median progression-free survival was 2.8 months longer and the risk of disease progression or death was 42% lower with olaparib monotherapy than with standard therapy.
Funded by: AstraZeneca; OlympiAD
ClinicalTrials.gov number: NCT02000622
DOI: 10.1056/NEJMoa1706450