乳腺癌易感基因突变女性的乳腺癌、卵巢癌、对侧乳腺癌风险
对乳腺癌易感基因(BRCA1、BRCA2)突变女性进行最佳临床管理,有赖于对不同年龄的癌症风险及其影响因素进行精准评估,这些数据可被用于评估相关预防措施对癌症风险的降低程度,并为决定什么年龄开始癌症筛查提供依据。
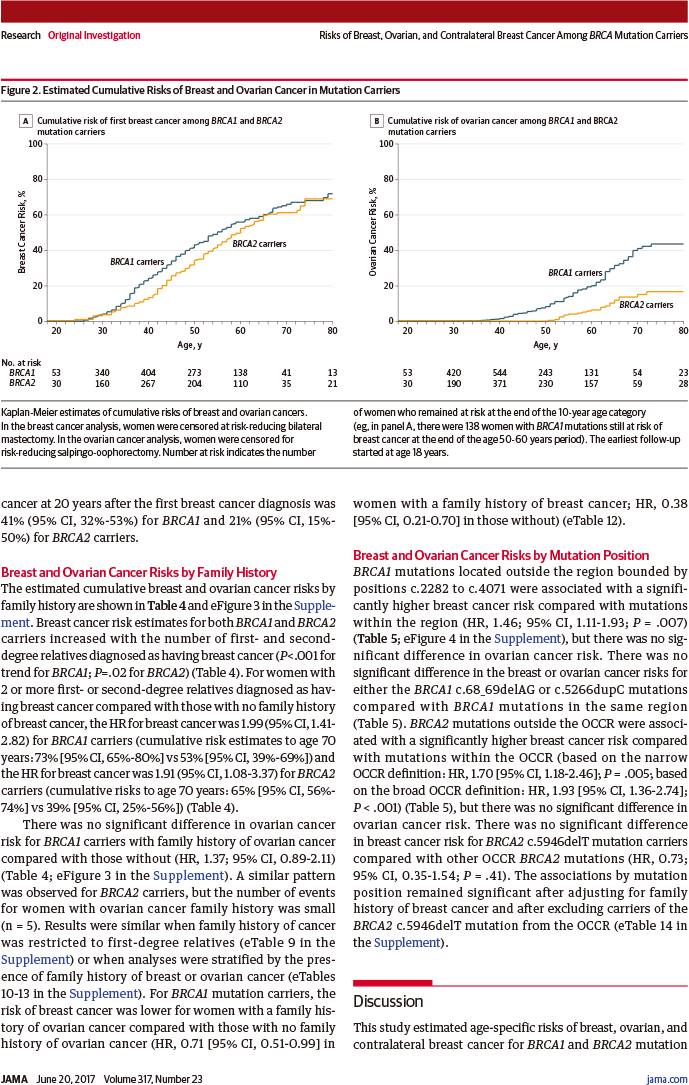
2017年6月20日,《美国医学会杂志》正式发表英国剑桥大学、威康基金会桑格研究所、华威大学、澳大利亚墨尔本大学、彼得麦卡伦癌症中心、圣文森特医院、维多利亚抗癌协会、荷兰癌症研究所、阿姆斯特丹大学、法国国家健康与医学研究院、居里研究所、巴黎工程技术大学、巴黎文理研究大学、美国犹他大学、哥伦比亚大学的大样本队列研究报告,评估了突变携带女性发生乳腺癌、卵巢癌、对侧乳腺癌的年龄相关风险,以及癌症家族史、突变位点对该风险的影响,结果发现BRCA1、BRCA2携带女性至80岁时,乳腺癌累积风险分别为72%、69%,卵巢癌风险分别为44%、17%,癌症风险随癌症家族史和突变位点而不同。
该研究于1997~2011年从国际BRCA1/2突变携带者队列、乳腺癌家族登记队列、凯思琳坎宁安家族性乳腺癌研究基金会联盟队列入组BRCA突变携带女性9856例,其中94%来自癌症家族遗传门诊、6%来自人群研究,BRCA1突变者6036例、BRCA2突变者3820例,入组时无癌症者5046例、已有单侧乳腺癌或卵巢癌者4810例。
数据主要来自大样本全国研究,例如英国的EMBRACE研究、荷兰的HEBON研究、法国的GENEPSO研究。
随访至2013年12月,中位随访5年:
-
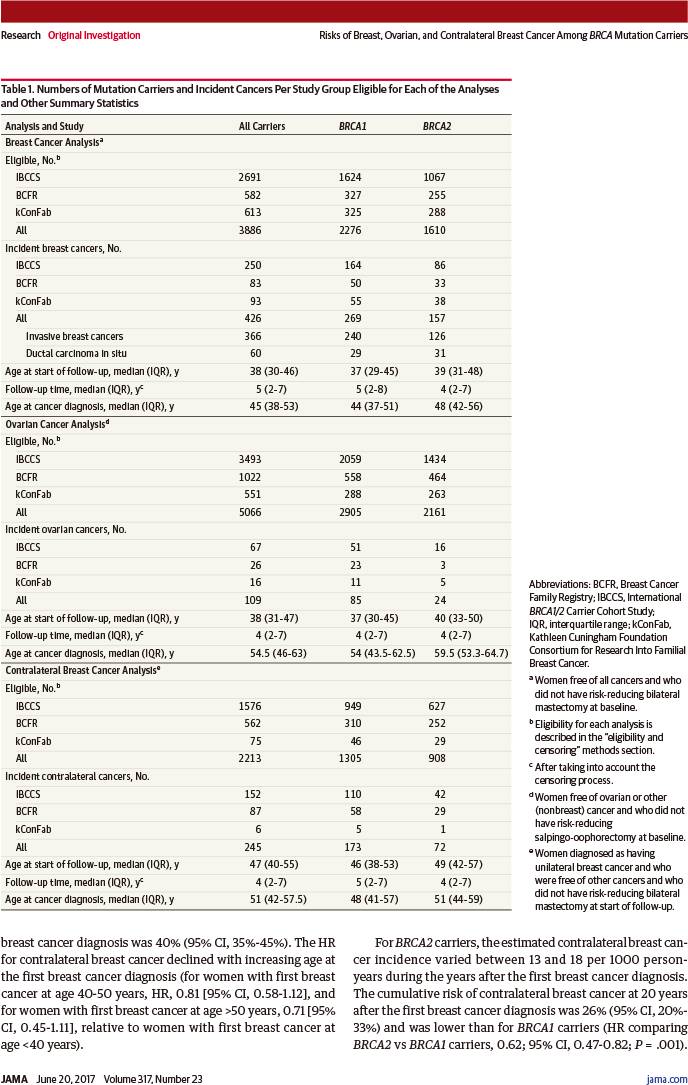
符合乳腺癌分析条件3886例(中位年龄38岁,四分位距:30~46岁)
-
符合卵巢癌分析条件5066例(中位年龄38岁,四分位距:31~47岁)
-
符合对侧乳腺癌分析条件2213例(中位年龄47岁,四分位距:40~55岁)
-
诊断乳腺癌426例
-
诊断卵巢癌109例
-
诊断对侧乳腺癌245例
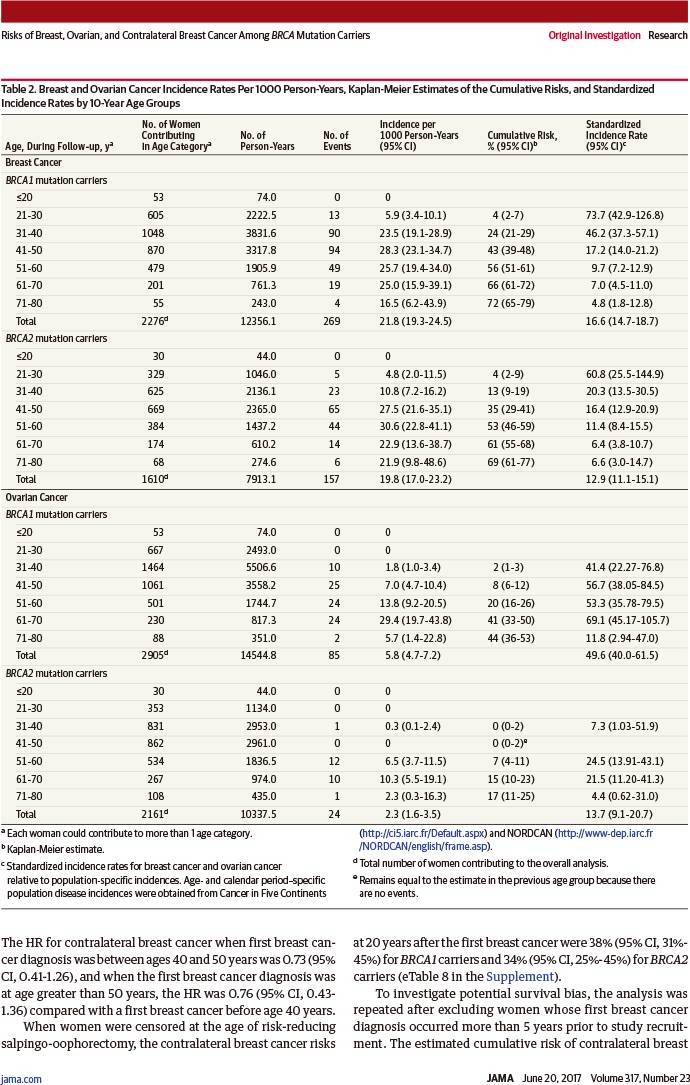
至80岁时乳腺癌累积风险:
-
BRCA1突变者:72%(95%置信区间:65%~79%)
-
BRCA2突变者:69%(95%置信区间:61%~77%)
乳腺癌发病率从成年后早期迅速增加,直至:
-
BRCA1突变者:30~40岁
-
BRCA1突变者:40~50岁
然后保持相似恒定的发病率(每年20~30‰)直至80岁。
至80岁时卵巢癌累积风险:
-
BRCA1突变者:44%(95%置信区间:36%~53%)
-
BRCA2突变者:17%(95%置信区间:11%~25%)
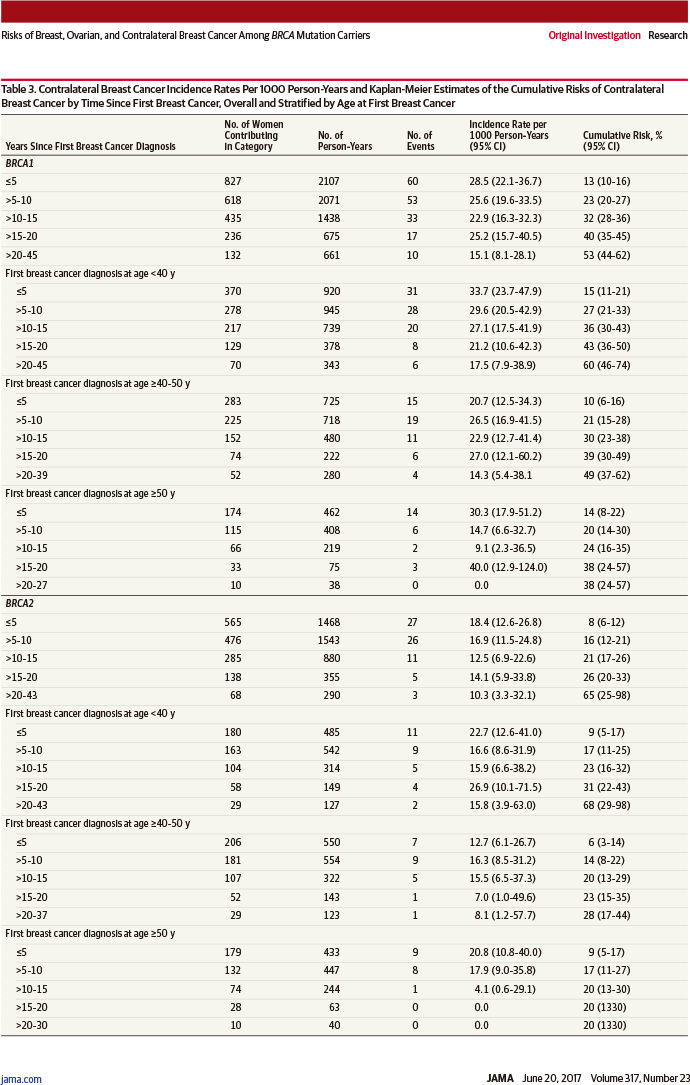
单侧乳腺癌诊断20年后对侧乳腺癌累积风险:
-
BRCA1突变者:40%(95%置信区间:35%~45%)
-
BRCA2突变者:26%(95%置信区间:20%~33%)
-
风险比0.62(95%置信区间:0.47~0.82,相差P=0.001)
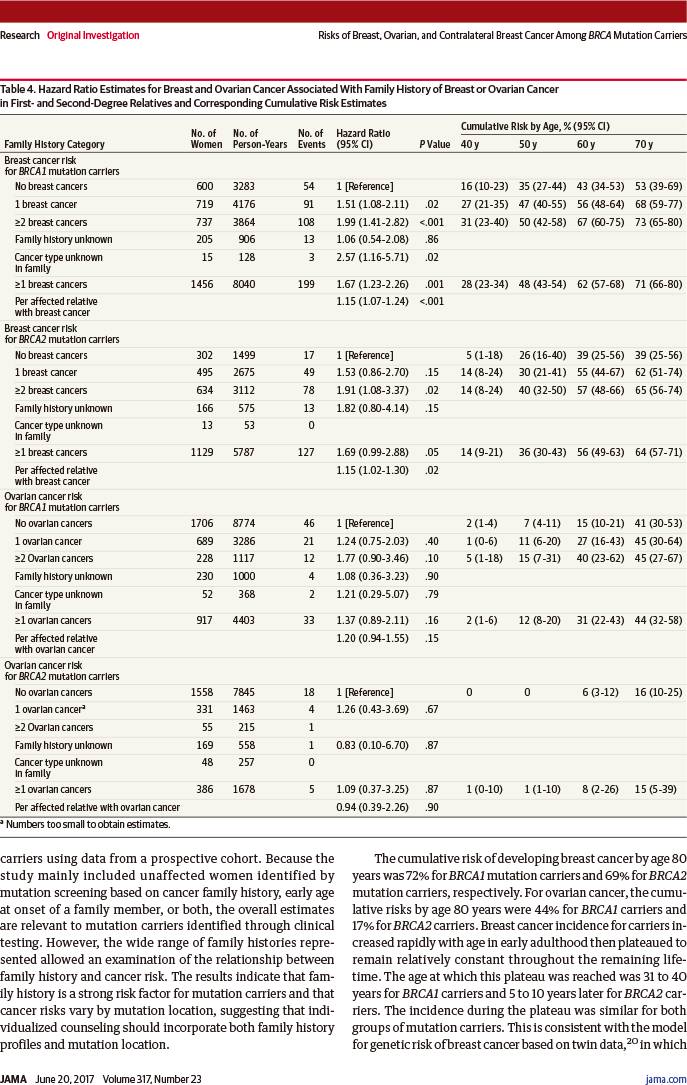
乳腺癌风险随着一级亲属(父母、子女、同父母的兄弟姐妹)和二级亲属(叔、伯、舅、姑、姨、祖父母、外祖父母)被诊断乳腺癌人数增加而增加,累及人数≥2与0相比:
-
BRCA1突变者:风险比1.99(95%置信区间:1.41~2.82,趋势P<0.001)
-
BRCA2突变者:风险比1.91(95%置信区间:1.08~3.37,趋势P=0.02)
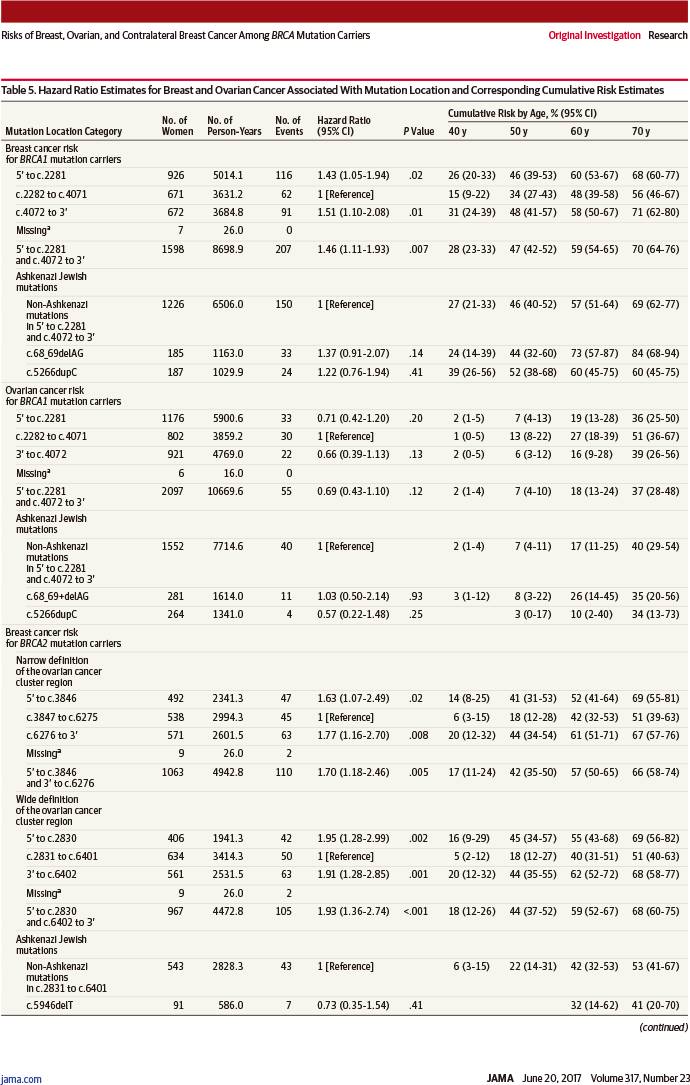
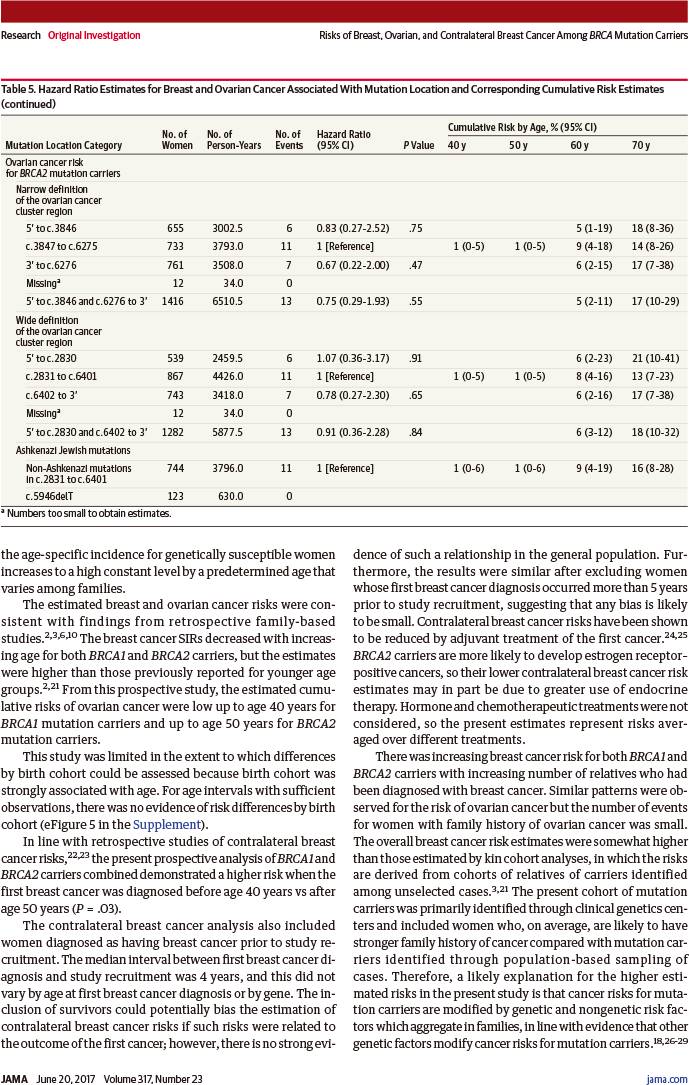
若突变位于以下位点,则乳腺癌风险显著增加:
-
BRCA1突变者:c.2282~c.4071(风险比1.46,95%置信区间:1.11~1.93,P=0.007)
-
BRCA1突变者:c.2831~c.6401(风险比1.93,95%置信区间:1.36~2.74,P<0.001)
因此,这些结果表明,癌症家族史是基因突变携带女性的癌症高风险因素,而癌症风险因突变位点而异,提示个体化遗传咨询应该结合家族史档案和基因突变位点。
本研究的局限性:虽然基因突变携带女性的癌症风险因癌症家族史而异,但是本研究的样本并非来自无癌症女性人群筛查,故该结果可能无法直接应用于这些女性。
JAMA. 2017 Jun 20;317(23):2402-2416.
Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers.
Karoline B. Kuchenbaecker; John L. Hopper; Daniel R. Barnes; Kelly-Anne Phillips; Thea M. Mooij; Marie-José Roos-Blom; Sarah Jervis; Flora E. van Leeuwen; Roger L. Milne; Nadine Andrieu; David E. Goldgar; Mary Beth Terry; Matti A. Rookus; Douglas F. Easton; Antonis C. Antoniou; BRCA1 and BRCA2 Cohort Consortium.
University of Cambridge, Cambridge, England; Wellcome Trust Sanger Institute, Cambridge, England; University of Melbourne, Melbourne, Australia; Peter MacCallum Cancer Centre, Melbourne, Australia; St Vincent's Hospital, Parkville, Australia; University of Melbourne, Parkville, Australia; Netherlands Cancer Institute, Amsterdam, the Netherlands; University of Amsterdam, Amsterdam, the Netherlands; University of Warwick, Coventry, England; Cancer Council Victoria, Melbourne, Australia; Inserm U900, Paris, France; Institut Curie, Paris, France; Mines ParisTech, Fontainebleau, France; PSL Research University, Paris, France; University of Utah, Salt Lake City, Utah; Columbia University, New York, New York.
This cohort study estimates age-specific risks of breast, ovarian, and contralateral breast cancer among carriers of BRCA1 and BRCA2 mutations and evaluates risk modification by family cancer history and location of the mutation within the BRCA gene.
QUESTION: What are the breast and ovarian cancer risks for BRCA1 and BRCA2 mutation carriers and are they related to family history of cancer and mutation position?
FINDINGS: From a prospective cohort of 9856 mutation carriers, mainly ascertained through cancer genetic clinics, the cumulative breast cancer risk to age 80 years was 72% for BRCA1 and 69% for BRCA2 carriers. The cumulative ovarian cancer risk to age 80 years was 44% for BRCA1 and 17% for BRCA2 carriers. Cancer risks differed by cancer family history and mutation position.
MEANING: These findings provide cancer risk patterns based on BRCA status using prospective data. Family history and mutation position are important additional variables in risk assessment.
IMPORTANCE: The clinical management of BRCA1 and BRCA2 mutation carriers requires accurate, prospective cancer risk estimates.
OBJECTIVES: To estimate age-specific risks of breast, ovarian, and contralateral breast cancer for mutation carriers and to evaluate risk modification by family cancer history and mutation location.
DESIGN, SETTING, AND PARTICIPANTS: Prospective cohort study of 6036 BRCA1 and 3820 BRCA2 female carriers (5046 unaffected and 4810 with breast or ovarian cancer or both at baseline) recruited in 1997-2011 through the International BRCA1/2 Carrier Cohort Study, the Breast Cancer Family Registry and the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer, with ascertainment through family clinics (94%) and population-based studies (6%). The majority were from large national studies in the United Kingdom (EMBRACE), the Netherlands (HEBON), and France (GENEPSO). Follow-up ended December 2013; median follow-up was 5 years.
EXPOSURES: BRCA1/2 mutations, family cancer history, and mutation location.
MAIN OUTCOMES AND MEASURES: Annual incidences, standardized incidence ratios, and cumulative risks of breast, ovarian, and contralateral breast cancer.
RESULTS: Among 3886 women (median age, 38 years; interquartile range [IQR], 30-46 years) eligible for the breast cancer analysis, 5066 women (median age, 38 years; IQR, 31-47 years) eligible for the ovarian cancer analysis, and 2213 women (median age, 47 years; IQR, 40-55 years) eligible for the contralateral breast cancer analysis, 426 were diagnosed with breast cancer, 109 with ovarian cancer, and 245 with contralateral breast cancer during follow-up. The cumulative breast cancer risk to age 80 years was 72% (95% CI, 65%-79%) for BRCA1 and 69% (95% CI, 61%-77%) for BRCA2 carriers. Breast cancer incidences increased rapidly in early adulthood until ages 30 to 40 years for BRCA1 and until ages 40 to 50 years for BRCA2 carriers, then remained at a similar, constant incidence (20-30 per 1000 person-years) until age 80 years. The cumulative ovarian cancer risk to age 80 years was 44% (95% CI, 36%-53%) for BRCA1 and 17% (95% CI, 11%-25%) for BRCA2 carriers. For contralateral breast cancer, the cumulative risk 20 years after breast cancer diagnosis was 40% (95% CI, 35%-45%) for BRCA1 and 26% (95% CI, 20%-33%) for BRCA2 carriers (hazard ratio [HR] for comparing BRCA2 vs BRCA1, 0.62; 95% CI, 0.47-0.82; P=.001 for difference). Breast cancer risk increased with increasing number of first- and second-degree relatives diagnosed as having breast cancer for both BRCA1 (HR for ≥2 vs 0 affected relatives, 1.99; 95% CI, 1.41-2.82; P<.001 for trend) and BRCA2 carriers (HR, 1.91; 95% CI, 1.08-3.37; P=.02 for trend). Breast cancer risk was higher if mutations were located outside vs within the regions bounded by positions c.2282-c.4071 in BRCA1 (HR, 1.46; 95% CI, 1.11-1.93; P=.007) and c.2831-c.6401 in BRCA2 (HR, 1.93; 95% CI, 1.36-2.74; P<.001).
CONCLUSIONS AND RELEVANCE: These findings provide estimates of cancer risk based on BRCA1 and BRCA2 mutation carrier status using prospective data collection and demonstrate the potential importance of family history and mutation location in risk assessment.
DOI: 10.1001/jama.2017.7112